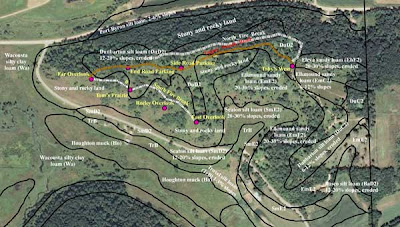
One of the glories of Pleasant Valley Conservancy is the large bur oak that is near our driveway. This tree is one of our landmarks, and has acted as a sentinel in our restoration work. When we first began restoration, this tree was invisible behind a screen of elms and buckthorns. Once these had been cleared away, we had a veritable "buckthorn desert" for several years until the buckthorn toxin dissipated in the soil. The seeds we planted around it the first few years did not "take". Finally, about year three, we started seeing results.
Two problems remained, which we finally were able to deal with this year: smooth brome and buckthorn.
Smooth brome (
Bromus inermis) is a nonnative clonal grass that is widely planted in southern Wisconsin farms and is a prolific seed producer. It is able to colonize any bare area and establish a green mat that keeps out native grasses. By the third year after clearing, it had become well established in this area, and persisted despite annual burns.
If a prairie greens up quickly after a spring burn, you can be almost certain that it is due to smooth brome. Once we realized this, we started getting serious about smooth brome control.
The "trick" to eliminate smooth brome is an early spring glyphosate treatment, before any native plants have appeared. Remember: if the plant is not above ground when you spray with glyphosate, it won't be affected because the herbicide is inactivated by soil particles. We had already used this method to eliminate smooth brome on the Pleasant Valley Road cut, and in the Barn, Valley, Ridge, and Crane Prairies. Timing is critical, so spraying should be done when the grass leaves are about 4 inches tall.
At the big burn oak, smooth brome was only a problem south of the tree, an area of full sun. Because the smooth brome area around the big bur oak was fairly small, Kathie and I used backpack sprayers. It took us about an hour to spray the area. Since the area had been seeded with prairie plants some years ago, we did not have to wait long to see results. By the end of July Indian grass was flourishing throughout, interspersed with many good prairie plants, including cream gentian, compass plant, rosin weed, cone flowers, etc.
In the photo here, taken from the uphill side of the oak, you can see below the tree a red-brown carpet of Indian grass. Very nice.
The second problem here is more difficult to handle: buckthorn! Even though this area has been burned every year for the past 10 years, small buckthorn plants persist. (Don't believe those who tell you that you can eliminate buckthorn by burns alone!) These plants are not from the seed bank (which was exhausted some years ago), but from dormant rootstock that is able to persist. In the area around the oak, there were scattered about three dozen small buckthorn plants, with stems about knee to waist high. These plants were top-killed by the spring burn, but dormant buds sprouted. There are usually from 2-4 green leafy stems at each plant, quite visible now that many of the native species have started to senesce. I am controlling them by careful basal bark treatment with triclopyr (Garlon 4) in oil. This is slow, tedious work, but effective.
(See this web site for more on buckthorn eradication.)With so many good plants present, it is important to use great care when spraying an herbicide such as Garlon.
See my earlier blog post for details.

 On Tuesday, Kathie and I collected quite a bit of zig-zag goldenrod (Solidago flexicaulis), an interesting woodland/savanna species. This is one of the latest blooming species of goldenrod, and its seeds are just now ready to collect. Strange that it is also one of the earliest goldenrods to appear in the spring. It remains a low, vegetative plant all summer and does not begin to flower until late August/early September.
On Tuesday, Kathie and I collected quite a bit of zig-zag goldenrod (Solidago flexicaulis), an interesting woodland/savanna species. This is one of the latest blooming species of goldenrod, and its seeds are just now ready to collect. Strange that it is also one of the earliest goldenrods to appear in the spring. It remains a low, vegetative plant all summer and does not begin to flower until late August/early September. In contrast to most goldenrods, the flowers of zig-zag goldenrod are formed in the axils of the leaves, where they remain relatively inconspicuous. Of course, the seeds also form in the axils of the leaves, making collecting them a bit of a challenge.
In contrast to most goldenrods, the flowers of zig-zag goldenrod are formed in the axils of the leaves, where they remain relatively inconspicuous. Of course, the seeds also form in the axils of the leaves, making collecting them a bit of a challenge.